Research Approval Pathway and Forms
Welcome Researchers,
Thank you for your interest in conducting research at Sault Area Hospital (SAH). SAH encourages and supports research conducted by healthcare workers in all disciplines/areas. Such research will benefit our patients/clients as well as the scientific community as a whole.
See the below recommended process for obtaining research approvals at SAH. We have also created the handy SAH Research Approval Checklist (link below) as a guidance document for approvals during the lifecycle of your research project.
See our contact information at the bottom of this page should you need any assistance. We aim to make this process as easy and accessible as possible.
Sincerely,
SAH Research Office
What do I need to conduct research at SAH?
We understand that the research process is not always linear. This is our suggested pathway for obtaining approvals. They can be done in any order. However, you need BOTH hospital approval and REB approval to begin your research.
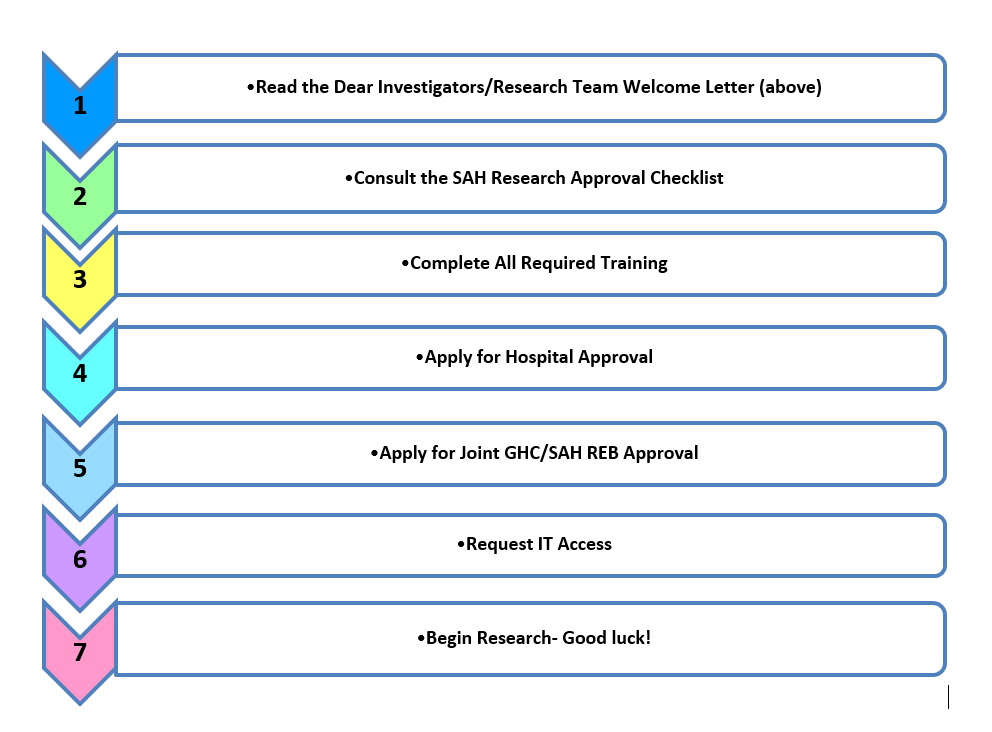
Please read the above information.
Open, print, and read the SAH Research Approval Checklist. It details all administrative requirements necessary to work with SAH’s patients, people, and data. Use this as a guidance document before you begin your study (i.e. to seek required approvals), throughout your study, and at the conclusion of your study.
Complete all required training.
For non-interventional or retrospective chart reviews:
Principal Investigator and all members of the research team: Complete your TCPS2 training and obtain your certificate. You will print and attach a copy with your REB application.
For Clinical Trials:
Principal Investigator and all members of the research team: Complete your Good Clinical Practice training and obtain your certificate. You will print and attach a copy with your REB application. Contact the Research Office for instructions on how to access this program: research@sah.on.ca
For questions regarding the initiation of a Clinical Trial, contact Natalie Walde: research@sah.on.ca
Note: if you are conducting a Clinical Trial, Health Canada approval may be required through a Clinical Trial Application.
Standard Operating Procedures (SOPs)
Principal Investigator and all members of the research team: you are required to train on SOPs at the Institution where research is being conducted. If research is being conducted at SAH, please email research@sah.on.ca
If research is being conducted at GHC, please contact the GHC Privacy Officer through the GHC Switchboard at 705-759-1234.
Note: Reviewing of SOPs can be done after Institutional/REB approval and will be kept on file. SOPs update bi-annually at each site; Investigators and research teams should remain informed about those changes and sign a training document each time they are reviewed. SOPs are copyrighted and cannot be shared (photocopied/scanned/printed) with anyone outside of SAH or GHC.
Apply for Hospital Approval
For help to determine which documents you must complete for your study, contact research@sah.on.ca
For research projects requiring access to an SAH patient’s/client’s Personal Health Information (PHI) found in medical charts:
•Request for Departmental Approval—Health Records Form
Debbie Sacco (Manager, Health Records) at research@sah.on.ca
- Request for Departmental Approval—Feasibility & Analytics Form
Vladimir Yasenovskiy (Manager, Feasibility & Analytics) at research@sah.on.ca
For research projects seeing patients/clients or clinicians, or requiring services from a specialty SAH department (such as the lab or pharmacy):
- Request for Departmental Approval—General
Administrative approval from manager/director of impacted department/unit
Contact research@sah.on.ca to find out which Manager/Director to contact for approval.
For research projects requiring access to an SAH patient’s/client’s Personal Health Information (PHI) found in medical charts:
•Data Sharing Agreement (DSA)
To request this document, as well as tips on how to complete it, contact research@sah.on.ca.
This document must be signed by the PI, any identified Research Institutions, and SAH prior to starting the study.
For questions regarding signatures/completion, PHIPPA, or language within the DSA, contact research@sah.on.ca.
Apply for REB Approval
- REB New Study Application
Contact REB@sah.on.ca for questions regarding this application or process.
All other REB forms and guidance documents can be accessed through the Research Ethics Board (GHC/SAH) page.
Once you’ve completed all required training and forms (including a fully executed Data Sharing Agreement) and you’ve received an REB Approval Letter, you may submit your IT request forms.
- Complete and submit all necessary IT forms (see below). You may request the forms through research@sah.on.ca.
IT Department Forms | Required for/by |
Network Access Request Form | Internal and External Staff by Manager/Coordinator |
Internet Access Form | If required/requested by the IT Department |
SAH Remote Access (Form A) – Manager Approval | External Staff by Manager/Coordinator with signing authority |
SAH Remote Access (Form B) – Individual VPN Access | External Staff by Manager/Coordinator with signing authority |
Confidentiality Agreement | External Staff by staff requesting access |
Privacy and Confidentiality Policy | Information purposes (no signature required) |
Continue referring to the SAH Research Approval Checklist. It guides you through the required REB forms to complete during your study and upon its completion. Good luck!
SAH Research Information
Request For Department Approval (RDA) Forms
Joint GHC/SAH Research Ethics Board
SAH Research Ethics Board
REB@sah.on.ca
Clinical Trial Inquiries
Research Office
research@sah.on.ca
